Introduction: The Hypnotic Dance of Liquid Magnets
Few substances capture the eye quite like ferrofluids when they encounter a magnetic field. Their surface erupts into a mesmerizing array of spikes, troughs, and swirls, performing a seemingly impossible ballet. It feels like watching liquid defy its nature, pulled and shaped by an invisible force. This isn’t just a cool science demo you might see online; it’s the fascinating world of “liquid magnets.” Ferrofluids possess a truly unique dual identity: flowing like a liquid while responding powerfully to magnetic fields like a solid magnetic material. Born from the specific challenges of space exploration, these materials have since found surprising, often hidden, applications far beyond artistic displays, powering parts of the technology we use every day. You can learn more about materials science and its fascinating properties here.
What Exactly ARE Ferrofluids? (The Science Explained)
Understanding ferrofluids requires looking beyond simple mixtures. They are not merely magnetic powders mixed into a liquid that will eventually separate. Their stability and unique behavior stem from a precise composition, combining nanotechnology with fluid dynamics.
Beyond Just Iron Filings in a Jar
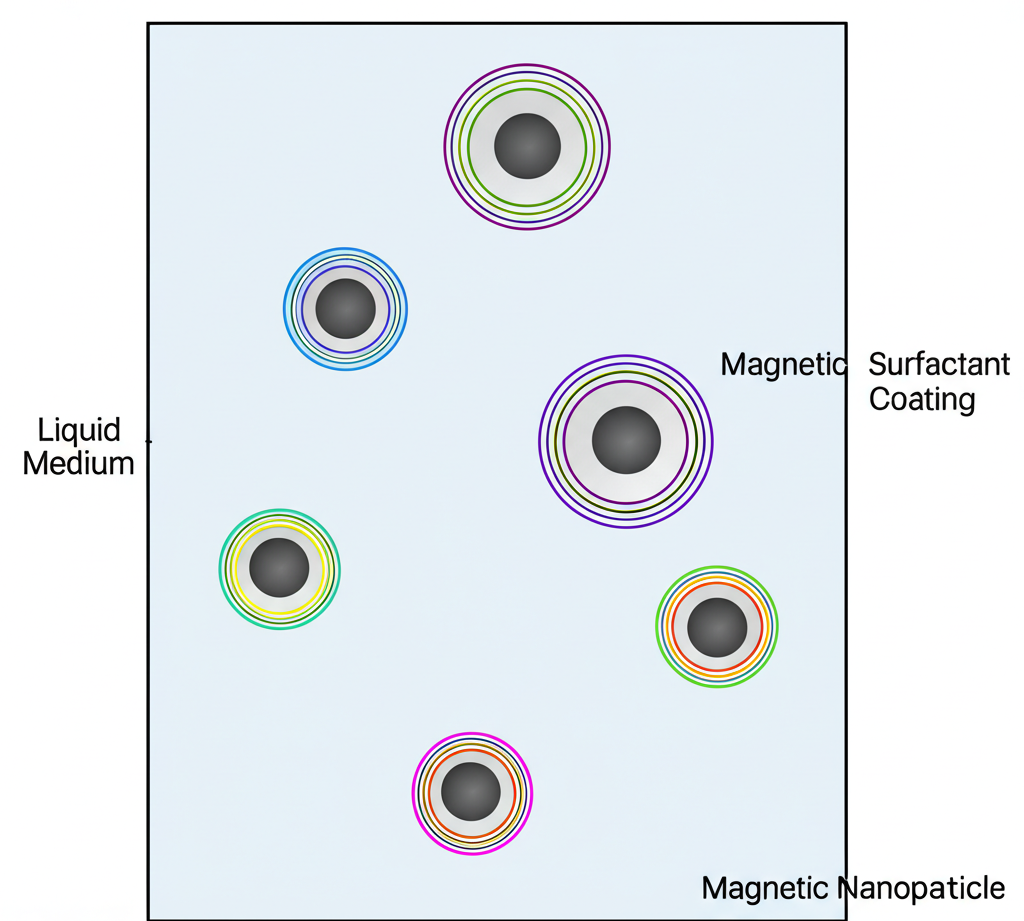
Ferrofluids are classified as stable colloidal suspensions. This means tiny solid particles are evenly dispersed within a liquid and remain suspended indefinitely, resisting settling over time. Achieving this stability requires three critical components working in concert:
- Magnetic Nanoparticles: These are the heart of the ferrofluid’s magnetic response. Typically made of strong magnetic materials like magnetite ($Fe_3O_4$), hematite, or other iron oxides, they are ground down to incredibly small sizes, usually between 3 and 15 nanometers. This nanoscale size is crucial because particles this small are constantly bombarded by the carrier liquid molecules (Brownian motion), which is strong enough to counteract gravity and magnetic attraction between particles, keeping them dispersed.
- Carrier Liquid: This is the fluid base, which can be water, oil (hydrocarbons), or various organic solvents. The choice of carrier liquid significantly influences the ferrofluid’s properties, such as its viscosity, boiling point, freezing point, and chemical compatibility, making it suitable for different environments and applications.
- Surfactant: This is arguably the most vital component for stability. Each magnetic nanoparticle is coated with a layer of surfactant molecules (like oleic acid, citric acid, or tetramethylammonium hydroxide). This coating acts as a barrier, preventing the tiny magnetic nanoparticles from clumping together (agglohttps://boostspan.com/bioluminescence-living-light-science/target=”_blank”merating), even when a strong magnetic field is applied. Without the surfactant, the particles would quickly aggregate and settle out of the suspension.
Together, these components create a homogeneous liquid that exhibits strong magnetic properties when exposed to a magnetic field, behaving in many ways like a single, large magnetic entity while retaining its fluid nature.
The Spiky Mystery: How They React to Magnets
The most visually arresting characteristic of ferrofluids is the formation of sharp peaks and valleys on their surface when a magnetic field is applied. This phenomenon is known as the Rosensweig instability.
The spikes appear because the magnetic field lines prefer to travel through the highly magnetic ferrofluid rather than the surrounding air. The magnetic force pulls the ferrofluid upwards along the field lines, which are often perpendicular to the surface near a magnet. This upward magnetic pull is countered by the forces trying to keep the surface flat: gravity (pulling the fluid down) and surface tension (trying to minimize the surface area). The spikes form when the magnetic force overcomes the restoring forces of gravity and surface tension at specific points, creating peaks along the magnetic field lines. The height and shape of these captivating spikes are directly influenced by the strength of the applied magnetic field and the specific properties of the ferrofluid, such as its magnetic strength and surface tension.
A History Born from the Space Race
Ferrofluids weren’t invented for art or entertainment. Their origin story is deeply rooted in the practical challenges of early space exploration, highlighting how specific engineering problems can drive materials science innovation.
The crucial problem faced by NASA engineers was how to reliably get liquid rocket fuel from the tanks to the engines in the zero-gravity environment of space. In the absence of gravity, liquids tend to float around or stick to the tank walls in unpredictable ways, making it difficult to ensure a continuous flow to the fuel pumps. They needed a way to control and position the liquid fuel using non-mechanical means.
This challenge led Dr. Stephen Papell at NASA’s Glenn Research Center (then Lewis Research Center) to develop the first ferrofluid in 1963. His innovative idea was to make the fuel itself responsive to magnetic fields. He achieved this by taking magnetic materials, grinding them to an extremely fine dust, and suspending these tiny particles in a liquid base using a surfactant.
Early attempts faced challenges in creating truly stable formulations where the magnetic particles wouldn’t settle or clump over time. Papell and other researchers iteratively refined the process, experimenting with different magnetic materials, particle sizes, carrier liquids, and surfactants to achieve the necessary stability and magnetic response. While the original concept for fuel management evolved, researchers soon recognized the broader potential of this novel material, paving the way for its use in various non-aerospace applications.
More Than Just Cool Visuals: Surprising Real-World Applications
Beyond the captivating science demos, ferrofluids have found their way into numerous practical applications across diverse industries, often working silently within everyday devices.
Acoustic Engineering (Speakers)
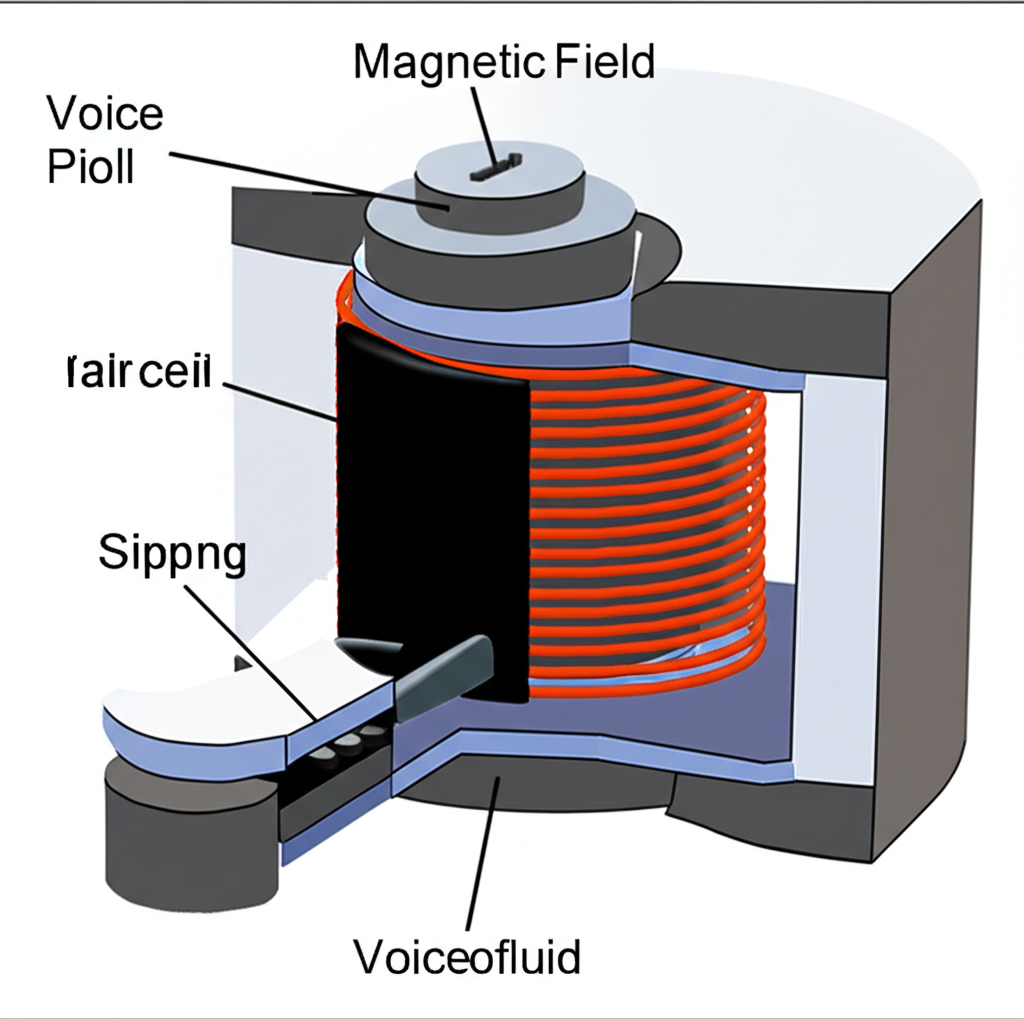
One of the most common, yet often unnoticed, uses of ferrofluid is in loudspeakers, particularly in tweeters (high-frequency drivers) and smaller full-range speakers. High-performance speakers face two main technical challenges: managing the significant heat generated by the voice coil as it rapidly vibrates and controlling the mechanical vibrations themselves to minimize distortion.
A tiny amount of ferrofluid is injected into the narrow gap between the speaker’s voice coil and the pole piece of the permanent magnet. Here, it serves two primary, critical functions. First, it acts as a highly efficient heat transfer medium. The heat generated by the voice coil is quickly transferred through the ferrofluid to the surrounding magnet structure, which acts as a large heat sink. This prevents the voice coil from overheating and failing. Second, the ferrofluid provides mechanical damping. Its viscosity helps to cushion the rapid, small movements of the voice coil, reducing unwanted resonances and vibrations. This improves power handling, lowers distortion, and extends the lifespan of the speaker driver.
Sealing and Bearings (Vacuum Seals, Hard Drives)
Ferrofluids excel at creating extremely reliable, low-friction seals, particularly in environments requiring high purity or vacuum conditions, such as equipment used in semiconductor manufacturing, medical devices, or scientific instruments.
In these applications, ferrofluid is held in place within a small gap by permanent magnets arranged around a rotating shaft. The ferrofluid forms a liquid barrier, effectively acting as a dynamic O-ring. This liquid seal prevents gases or contaminants from entering the sealed environment while allowing the shaft to rotate freely with minimal friction or wear, unlike traditional contact seals. A prime example is its use in computer hard drives. A ferrofluid seal is essential for preventing microscopic dust particles or moisture from entering the drive mechanism, where they could easily damage the sensitive magnetic platters and read/write heads, while allowing the central spindle supporting the platters to spin at high speeds.
Medical and Biomedical Potential (Research)
The magnetic properties of ferrofluids have opened exciting avenues for medical and biomedical research, although many applications are still in experimental or developmental stages.
One area is Magnetic Drug Delivery. Ferrofluid particles, potentially carrying therapeutic drugs attached to their surface or encapsulated within them, can be injected into the body. External magnetic fields can then be used to guide and concentrate these particles (and thus the drug) at a specific target site, such as a tumor. This approach aims to deliver a higher dose of medication precisely where it’s needed, minimizing systemic exposure and reducing side effects compared to traditional drug administration.
Another promising area is Magnetic Hyperthermia. Here, ferrofluid nanoparticles are injected into or near a tumor. Applying an alternating magnetic field causes the nanoparticles to heat up due to magnetic losses. This targeted heating can raise the temperature of the tumor cells to levels that are toxic, killing them with less damage to the surrounding healthy tissue than conventional radiation or chemotherapy.
Ferrofluid components can also be used as Contrast Agents for Imaging, particularly in Magnetic Resonance Imaging (MRI). Their magnetic properties enhance the contrast between different tissues or highlight specific structures or abnormalities, improving the diagnostic quality of the images.
Finally, Magnetic Separation utilizes ferrofluids in laboratory or diagnostic settings. Magnetic fields are used in conjunction with ferrofluids (often bound to specific biological targets) to isolate particular cells, proteins, DNA sequences, or other biomolecules from complex mixtures for analysis or purification.
Aerospace and Energy (Niche and Future Uses)
Beyond their current widespread uses, ferrofluids continue to be explored for niche and potentially groundbreaking applications in aerospace and energy.
Revisiting their origins, ferrofluids are still being researched for advanced aerospace applications, such as damping vibrations in sensitive spacecraft components or novel concepts for fluid management.
In the energy sector, experimental uses include enhancing heat transfer through thermomagnetic convection, where a temperature gradient in the fluid causes localized changes in magnetic susceptibility, driving fluid flow. There is also research into their potential use in magnetic refrigeration cycles, which offer a potentially more energy-efficient and environmentally friendly alternative to traditional gas compression refrigeration.
| Component | Role | Typical Material Examples |
|---|---|---|
| Magnetic Nanoparticle | Provides magnetic response | Magnetite, Hematite, Iron Oxides |
| Carrier Liquid | Suspension medium, determines fluid properties | Water, Hydrocarbons, Organic Solvents |
| Key Takeaway | Ferrofluids are stable colloidal suspensions, not simple mixtures. | Nanoparticle size (3-15 nm) is key. |
The Challenges and Future of Ferrofluids
Despite their impressive capabilities and diverse applications, ferrofluids still face challenges that limit broader adoption and drive ongoing research.
One significant hurdle can be Cost. Producing high-quality ferrofluids with consistent properties and excellent stability, particularly those designed for specialized or high-purity applications, remains more expensive than traditional fluids or sealing methods.
Maintaining long-term Stability is another key challenge. While modern ferrofluids are much more stable than early formulations, preventing the magnetic particles from eventually aggregating or settling under extreme conditions (like very high temperatures, prolonged exposure to very strong magnetic fields, or harsh chemical environments) can still be difficult depending on the specific application requirements. The Surfactant Selection is critical and complex; finding the perfect coating that is compatible with both the carrier liquid, the magnetic nanoparticle material, and the intended operational environment is essential for long-term performance. Furthermore, Scaling from a successful laboratory demonstration or small-scale production to mass manufacturing for widespread applications can present significant technical and economic hurdles.
Ongoing research and development efforts are focused on overcoming these limitations and expanding the potential of ferrofluids. Scientists are developing new types of magnetic nanoparticles with improved magnetic properties, novel compositions, or specific surface functionalities tailored for particular uses. Work is also being done to create ferrofluids with finely tunable properties, such as viscosity that changes predictably with temperature or magnetic field strength, opening up possibilities for smart fluids. Researchers are exploring entirely new application areas, including using ferrofluids for precise liquid manipulation in microfluidics devices, creating highly sensitive magnetic sensors, developing dynamic textures and feedback in haptics technology, and even crafting novel types of dynamic displays.
Potential future breakthroughs might involve integrating ferrofluids with other advanced materials or technologies, leading to entirely new functionalities. As production methods become more efficient and new formulations are discovered, we may see ferrofluid technology expand into even more hidden corners of our world, providing sophisticated solutions to problems we haven’t even fully defined yet.
Conclusion: The Enduring Fascination of Liquid Magnets
From a very specific problem of managing fuel in zero gravity, ferrofluids have embarked on a remarkable journey to become a versatile material with diverse industrial and burgeoning medical applications. They embody a fascinating paradox: a liquid that can be precisely controlled and shaped by the invisible hand of magnetism.
More than just visually stunning, this captivating substance is a sophisticated solution. Ferrofluids solve complex engineering challenges like dissipating heat in electronics, sealing sensitive environments with minimal friction, and offering new possibilities for targeted therapies within the body.
The story of ferrofluids reminds us how cutting-edge materials science, often driven by seemingly niche historical needs like the space race, can lead to technologies that quietly power essential parts of our modern world, proving that sometimes, the most useful innovations are found in the most unexpected places, like a bottle of liquid magnetism.
FAQ About Ferrofluids
Q1: Is ferrofluid just liquid metal?
A1: No, ferrofluid is not liquid metal like mercury. It’s a liquid (like water or oil) that contains incredibly tiny (nanoscale) solid particles of a magnetic material (like iron oxides) suspended throughout. The particles are coated with a surfactant to prevent them from clumping and settling, allowing the fluid to flow while responding to magnets.
Q2: Is ferrofluid dangerous to touch?
A2: It depends on the specific ferrofluid formulation. Many demonstration ferrofluids use an oil-based carrier liquid and are relatively safe for supervised interaction (like in science kits), but they can be messy and stain. Industrial or research ferrofluids might use different carrier liquids and chemicals that could be hazardous. It’s always best to avoid direct contact unless the specific fluid is known to be safe and handled appropriately.
Q3: Why does ferrofluid form spikes when a magnet is near?
A3: The spikes form because the magnetic force pulls the ferrofluid along the magnetic field lines, which are strongest and often perpendicular to the surface near the magnet. This upward magnetic pull is balanced by gravity and the fluid’s surface tension, which try to flatten the surface. The interaction of these forces creates the dramatic peaks in a phenomenon called the Rosensweig instability.
Q4: What are some common things that use ferrofluid?
A4: One of the most common hidden uses is in loudspeakers, where a small amount of ferrofluid cools the voice coil and provides damping to improve sound quality and lifespan. It’s also widely used in vacuum seals, particularly in computer hard drives, to prevent contamination while allowing internal components to rotate freely.
Q5: Can ferrofluids be used in the human body?
A5: Yes, researchers are actively exploring potential medical applications, but these are mostly experimental at this stage. Ideas include using ferrofluids for targeted drug delivery (guiding drugs to specific sites with magnets), magnetic hyperthermia (heating and killing cancer cells), or as contrast agents for medical imaging like MRI. Formulations for medical use must be biocompatible and sterile.

